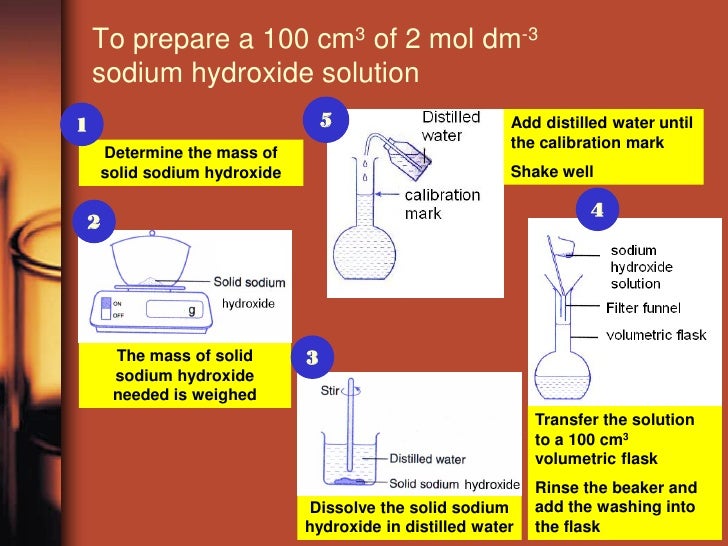
Making a Standard Solution
Description:
A standard solution is a solution who's concentration you know exactly. Standard solutions are often used by chemists and it is essential that they are made accurately. This lab went through the basics of making a standard solution with Sodium Hydrogen sulfate.
Standard Procedure for Making a Standard Solution:
- Calculate the mass of the solvent that is required to make the required amount of the wanted solution.
e.g. To make 250cm^(2) of 0.100 mol dm^(-3) we needed approximately 3.45g
-Weigh out the solvent, and pour it into a beaker. Weigh the weighing boat after you have poured out the solvent and subtract the traces from the previous recorded amount
e.g. Our final weight ended up being approximately 3.44
-Add a portion of distilled water to the beaker and stir until dissolved entirely
e.g. We used 100cm^(3)
-Pour the contents of the beaker into a volumetric flask using a funnel, and rinse the funnel and the beaker with distilled water. Fill the volumetric flask to the graduated mark.
e.g. For our experiment we used a 250cm^(3) volumetric beaker.
The same steps are illustrated in the picture below:
No comments:
Post a Comment