Experiment A
Boyle's law
Pressure is inversely proportional to volume.
We find this through the equation
PV=k
where k is a constant
We used this in a pressure-volume relationship for gases lab
By measuring the pressure in a syringe when we decreased the volume in the syringe. The pressure was measured by using a pressure sensor which we then got plotted into LoggerPro on a computer and choosing which volume we would have.

As the relationship was inversely proportional when we dotted the data we got a hyperbola.
There were several tasks concerning this lab, concidering the inversely proportional relationship, where if the volume is doubled what would happen to the pressure, and if the volume was halved, what would then happen to the pressure.
For example when the volume goes from 10 to 5 mL, the pressure will double.
Experiment B
Gay-Lussac's law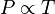
P/T=k
Gay-Lussac's law states that pressure is proportional to temperature.
Even though we didn't finish the second experiment, we still did the first part of it.
The experiment was about a pressure-temperature relationship and so when the temperature increased so would the pressure. This was proven by putting an 125 mL Erlenmeyer flask into 800 mL of water with different temperatures with the pressure sensor attached so , and then use LoggerPro to collect data and get a linear fit. The temperatures varied from ca. 48 C to 18 C.
No comments:
Post a Comment